Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube

SOLVED: The reaction between hydrochloric acid and sodium carbonate is represented by the unbalanced equation: HCl (aq) + Na2CO3 (aq)à NaCl (aq) + H2O (l) + CO2 (g) If 25.0 mL of

Lab 8 Sodium Carbonate or Sodium Bicarbonate? Objective To determine a compound to be either Na 2 CO 3 or NaHCO ppt download
_how-to-balance-na2co3-hcl-nacl-h2o-co2-sodium-carbonate-hydrochloric-acid.jpg)
How to Balance Na2CO3 + HCl = NaCl + H2O + CO2(Sodium carbonate + Hydrochloric acid) from co3 3nh Watch Video - HiFiMov.co

SOLVED: Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). This equation is balanced as written: 2HCl(aq)+Na2CO3(aq)→2NaCl(aq)+H2O(l)+CO2(g) Part A What volume of 1.75

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa

57.2g of hydrated sodium carbonate (Na<sub>2</sub>CO<sub>3</sub>.XH<sub>2</sub>O) were dissolved in water and the solution made to one litre. 20cm<sub>3</sub> of 0.5M HCl recated with 25cm<sub>3</sub> of...

Explain in detail the reaction between sodium carbonate with hydrochloride acid with figure - Brainly.in

We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com

When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid, the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm<sup>3</sup>). Calculate...
Write the balanced chemical equations for the following reactions: - Sarthaks eConnect | Largest Online Education Community

Molarity and Stoichiometry. 1.Calculate the number of grams of sodium carbonate that are required to react fully with mL of M HCl. Na 2 CO. - ppt download

What is observed when 2 mL of dilute hydrochloric acid is added to 1 g of sodium carbonate taken in a clean and dry test tube? Write chemical equation for the reaction involved.

Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid | Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Hello, Chemistry Enthusiasts! For today's

Sodium bicarbonate on heating decomposes to form sodium carbonate, CO2 and water. If 0.2 moles of sodium bicarbonate is completely decomposed, how many moles of sodium carbonate is formed?
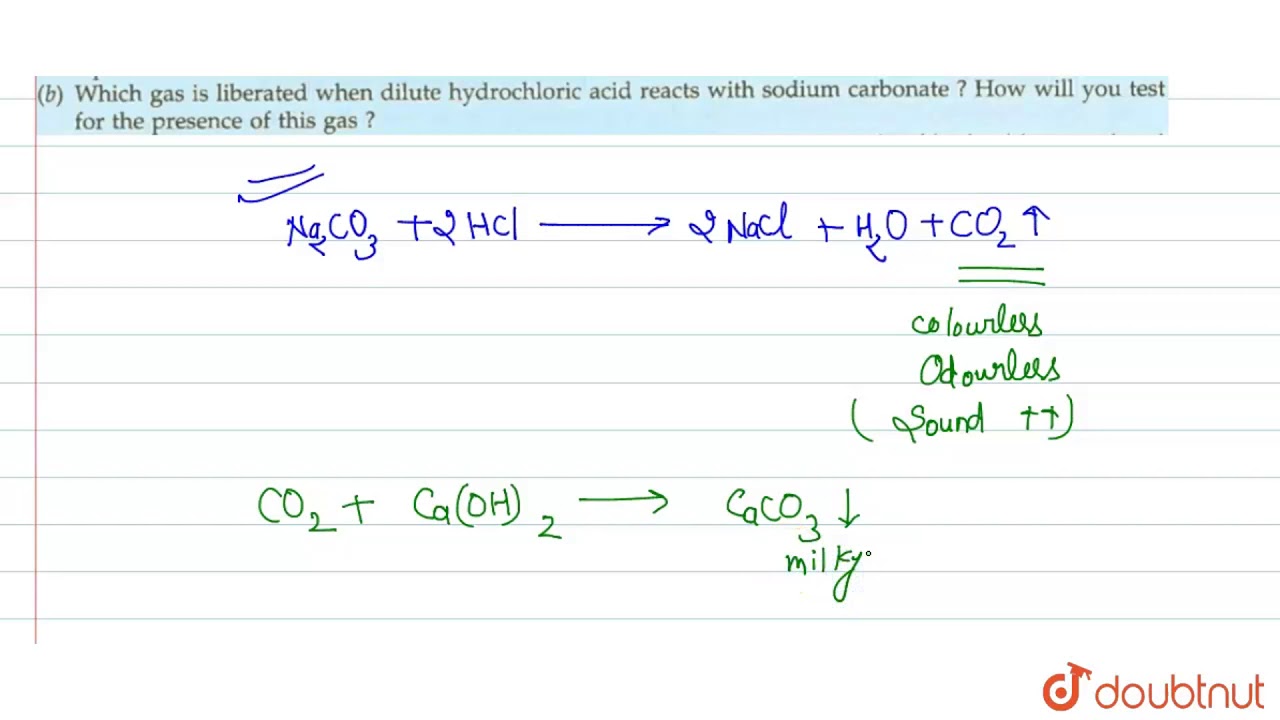
a). What happens when dilute hydrochloric acid is added to sodium carbonate? Write a balanced - YouTube

OneClass: If a solution of sodium bicarbonate is mixed with an equimolar solution of hydrochloric aci...