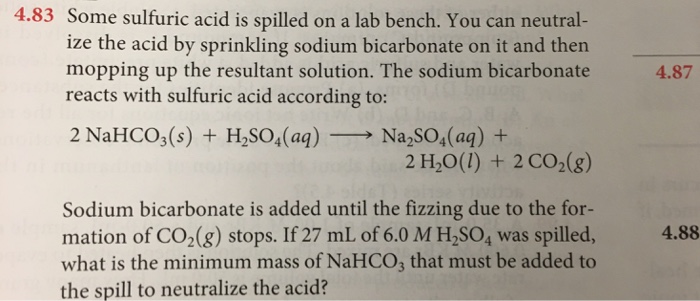
write balanced chemical equation for dilute sulphuric acid reacts with sodium carbonate . - Brainly.in
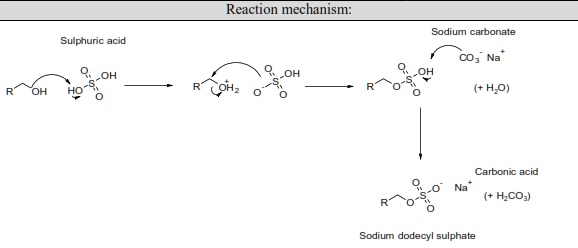
SOLVED: Reaction mechanism: Sodium carbonate Sulphurc acid CO3" Na COHz (+HJO) Carbonic acid HCO;) Sodium dodecyl sulphate

OneClass: 38. When sulfuric acid reacts with sodium hydrogen carbonate, three products form, two of w...

Question Video: Determining the Products Formed from the Reaction between Sodium Carbonate and Hydrochloric Acid | Nagwa
36. Assertion : Sodium Carbonate can be titrated against sulphuric acid by using methyl orange as indicator. Reason : The Volume of Sulphuric Acid required to produce the Colour change for the

Question Video: Determining the Concentration of Sulfuric Acid Via Titration with Sodium Carbonate | Nagwa
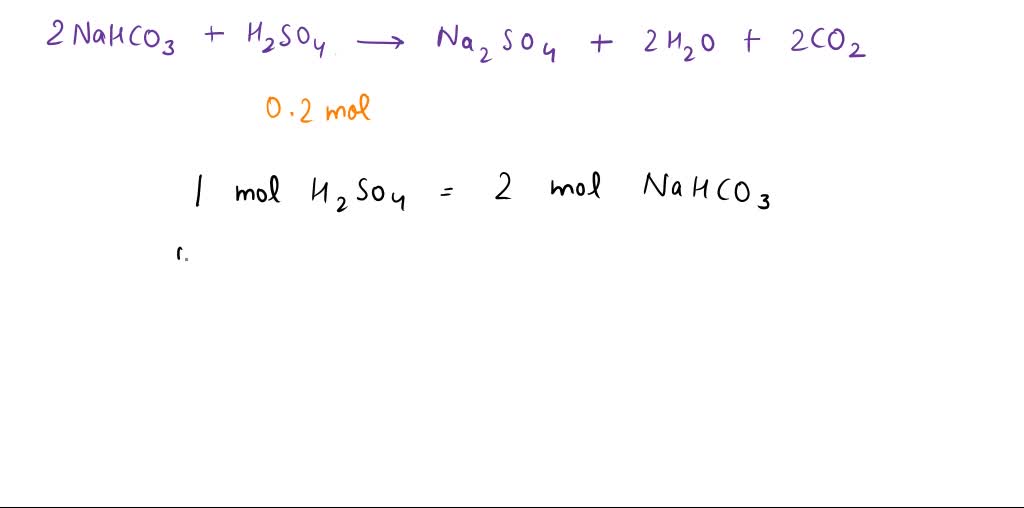
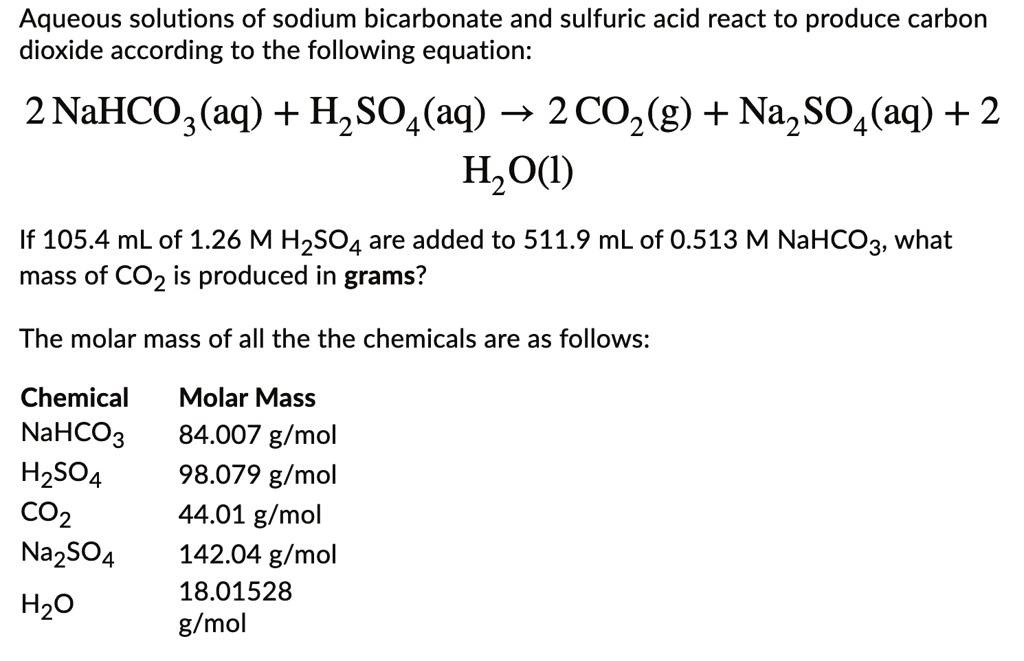
SOLVED: Sodium hydrogen carbonate (NaHCO3) reacts with sulfuric acid (H2SO4) to form sodium sulfate, carbon dioxide and water. What is the mass of sodium hydrogen carbonate required to neutralize 0.200 moles of
Making use only of substances given: dil. sulphuric acid, Sodium carbonate, Zinc, Sodium - Sarthaks eConnect | Largest Online Education Community
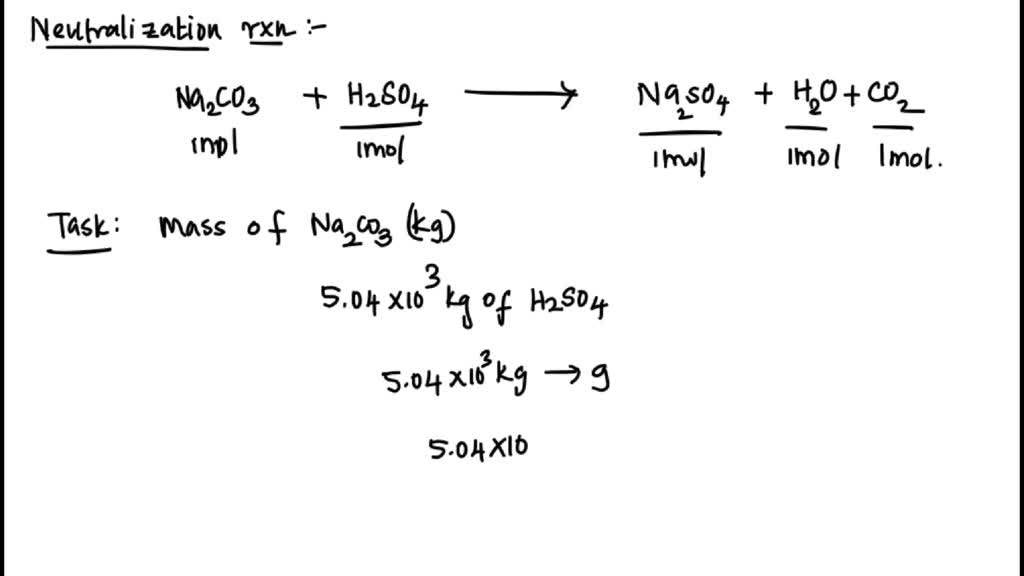
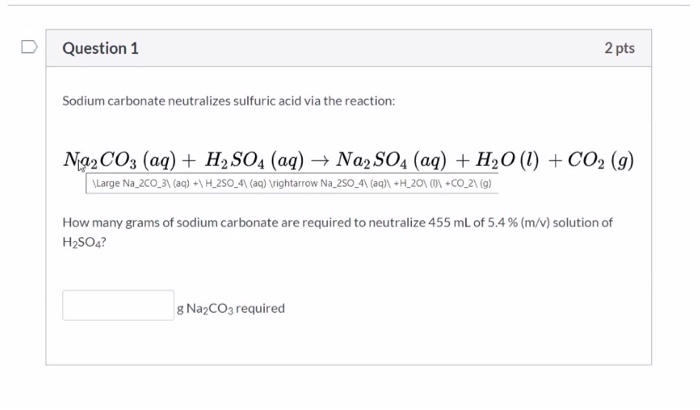
SOLVED: Sodium carbonate (Na2CO3Na2CO3) is used to neutralize the sulfuric acid spill. How many kilograms of sodium carbonate must be added to neutralize 5.04×103 kgkg of sulfuric acid solution?
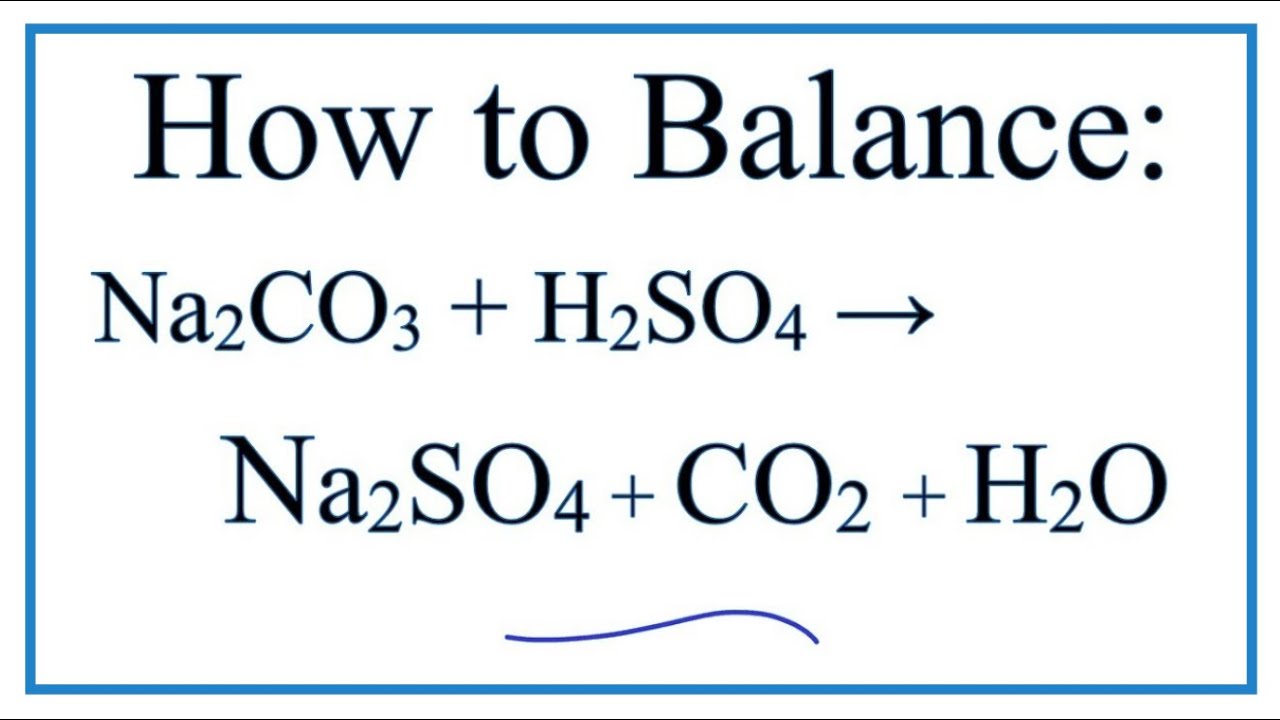
Sodium carbonate reacts with dil. H2SO4 to give the respective salt, water and carbon dioxide. - Sarthaks eConnect | Largest Online Education Community