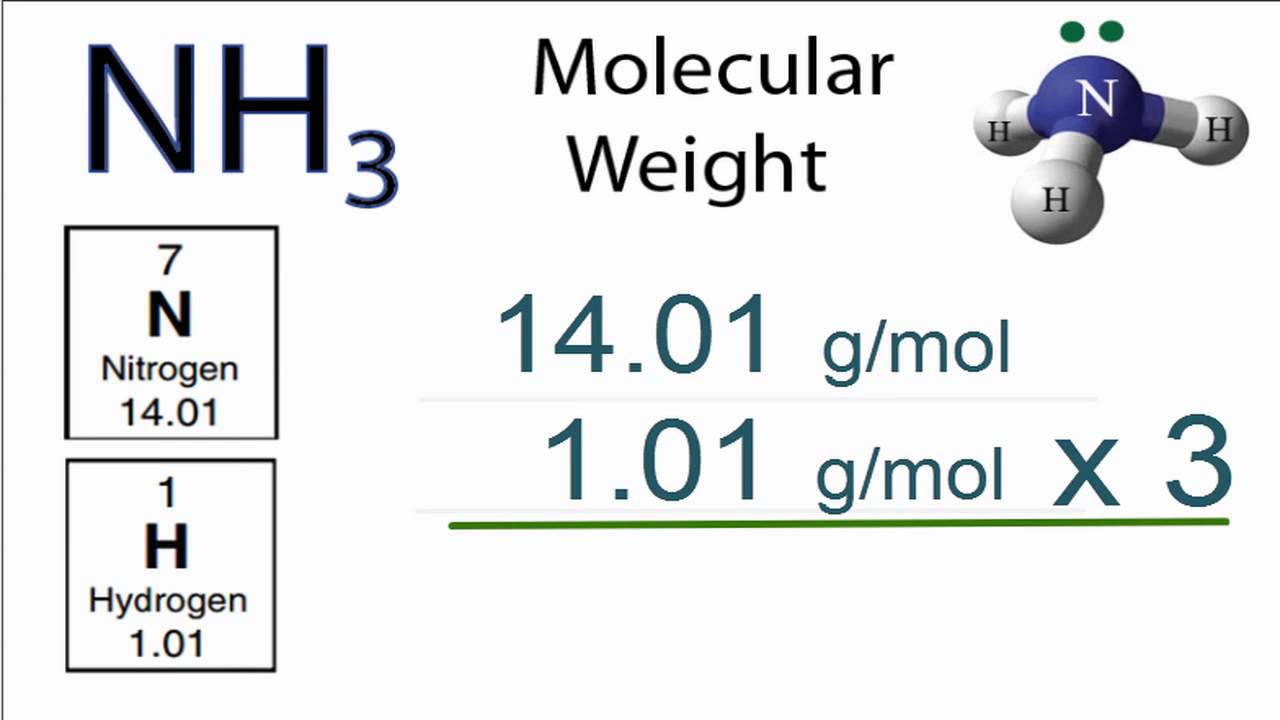
Calculate molecular weight Nitrogen|Molar mass of N2|Molecular weight Nitrogen |Nitrogen Molar mass - YouTube

Caffeine has a molecular weight of 194. If it contains 28.9 % by mass of nitrogen, number of atoms of nitrogen in one molecule of caffeine is

Nitrogen (N) solubility and N present in the soluble high molecular... | Download Scientific Diagram

Solve Q An oxide of nitrogen has a molecular weight 92 Find the total number of electrons in one - Chemistry - Chemical Kinetics - 12612235 | Meritnation.com
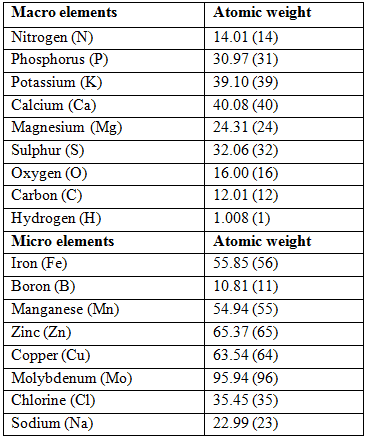
Commercial Hydroponic Farming | How to calculate nutrient content with atomic weight and molecular weight

Find the KE per kg of nitrogen molecule at 127^@C (molecular weight of nitrogen = 28, R = 8320 J//K mole K).

Caffiene has a molecular mass of 194. If it contains 28.9% by mass of nitrogen, number of atoms of nitrogen in one molecule of caffeine is :

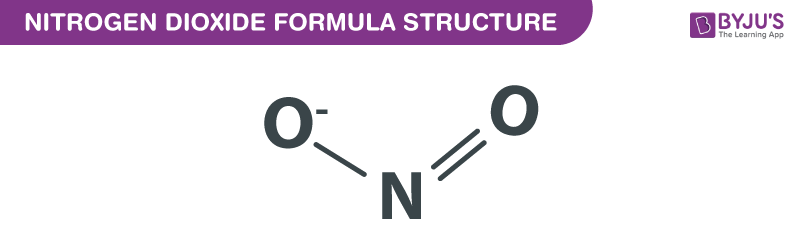
The molecular weight of an oxide of nitrogen is `30` .What is the number of electron is it ? - YouTube