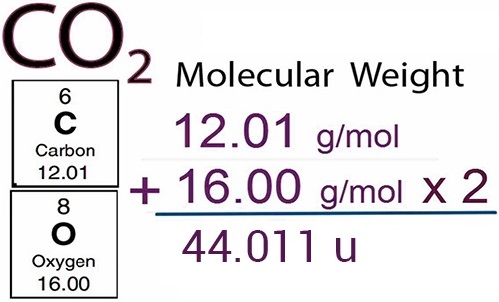
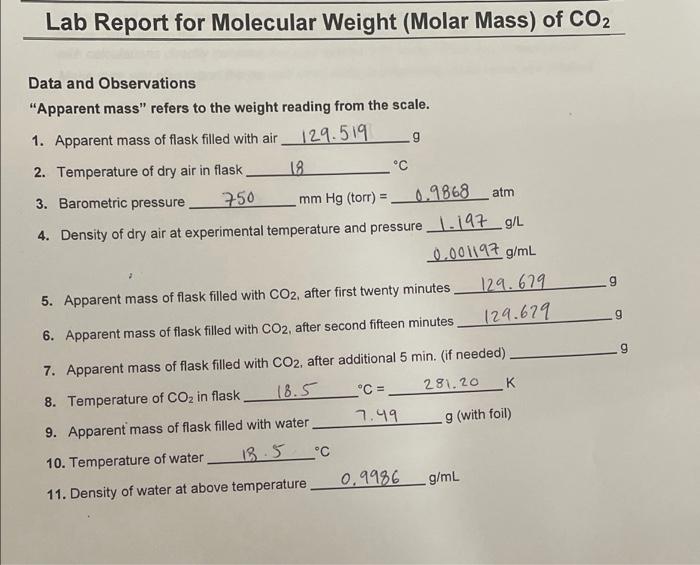
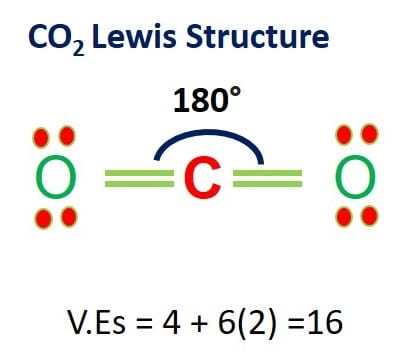
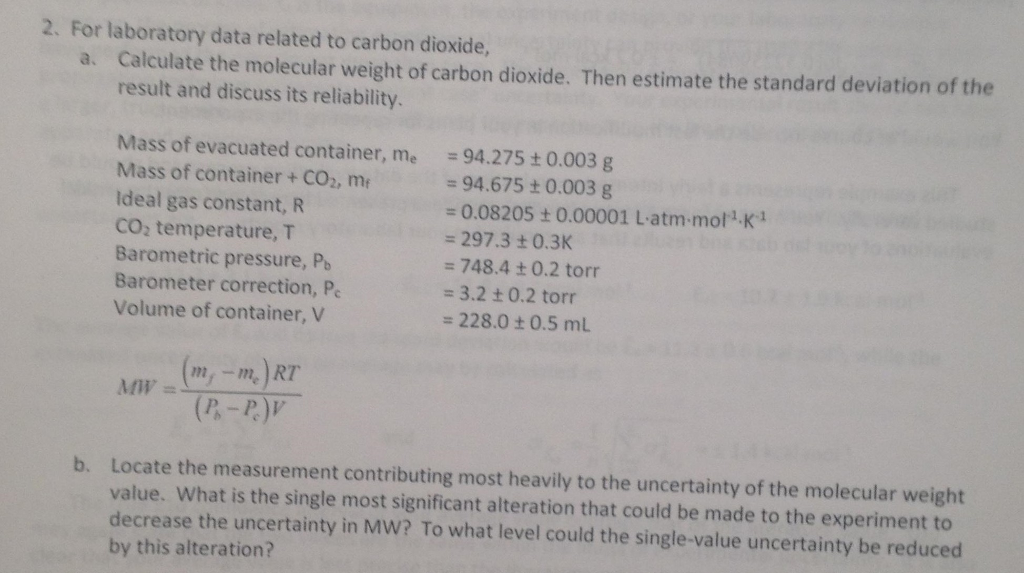
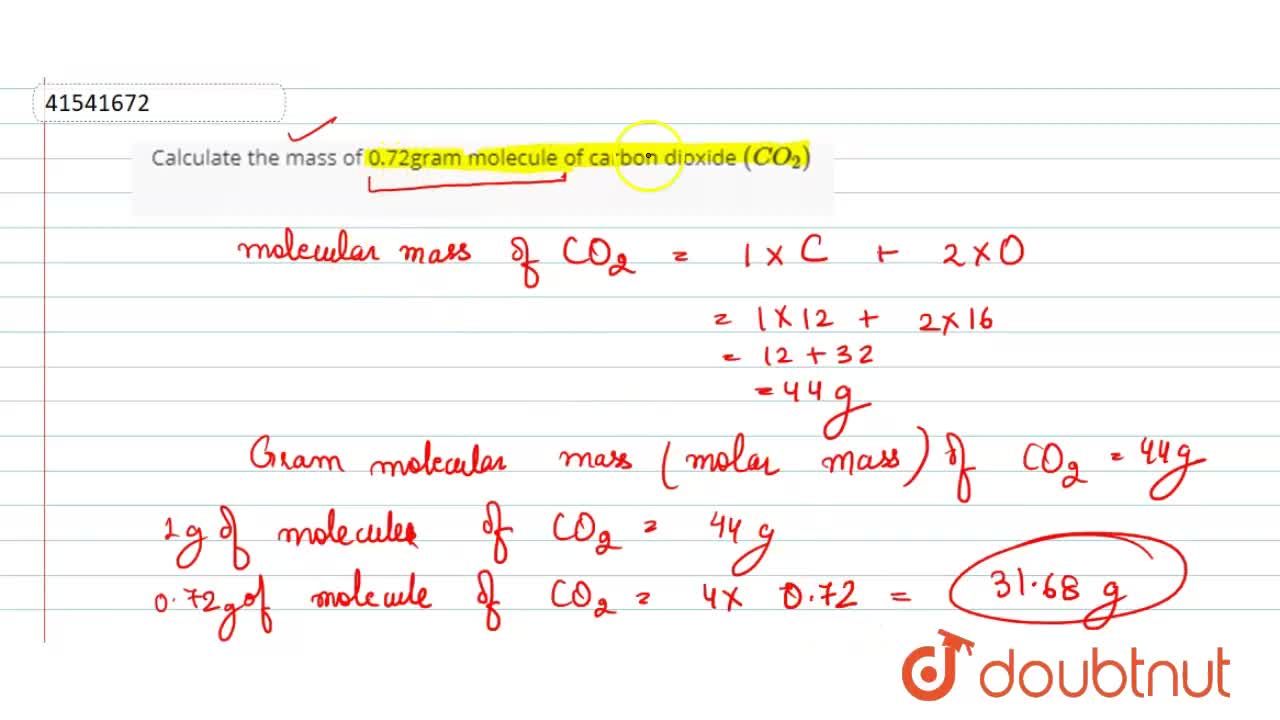
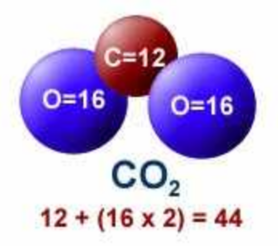
The molecular mass of CO2 is 44 amu and Avogadro's number is 6.02 × 10^23 . Therefore, the mass of one molecule of CO2 is:
![Calculate the molality of the solution containing 3g glucose dissolved in 30g of water. [Molar mass of glucose = 180] Calculate the molality of the solution containing 3g glucose dissolved in 30g of water. [Molar mass of glucose = 180]](https://dwes9vv9u0550.cloudfront.net/images/8902907/6b18b1a1-4e84-40d6-8c4f-456882950088.jpg)
Calculate the molality of the solution containing 3g glucose dissolved in 30g of water. [Molar mass of glucose = 180]

Why does carbon dioxide, with a molecular weight of 44.01, ascend in an atmosphere with a molecular weight of 28.97? - Quora

CO2 Lewis Structure: How to Draw the Dot Structure for CO2 | Chemical Bonding | Success in Chemistry

Take your periodic table out. What is atomic mass of Carbon Point where you can find it in the periodic table! 6 is atomic number not atomic mass Atomic. - ppt download

The Mole Calculating Formula/Molar Mass Calculate the molar mass of carbon dioxide, CO g + 2(16.00 g) = g One mole of CO 2 (6.02 x ppt download

Calculate the V.D. and molecular mass of CO2 is 200 ml of the gas at S.T.P. weighs 0.40g. (1l of H2 at - Brainly.in