Protons Neutrons Electrons Isotopes - Average Mass Number & Atomic Structure - Atoms vs Ions - YouTube

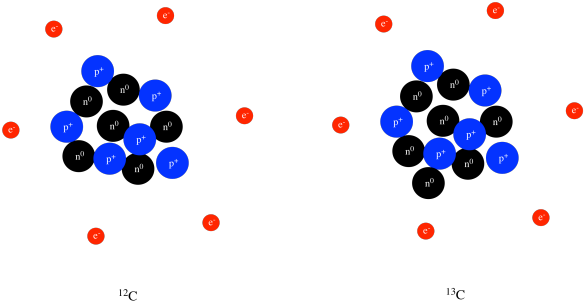
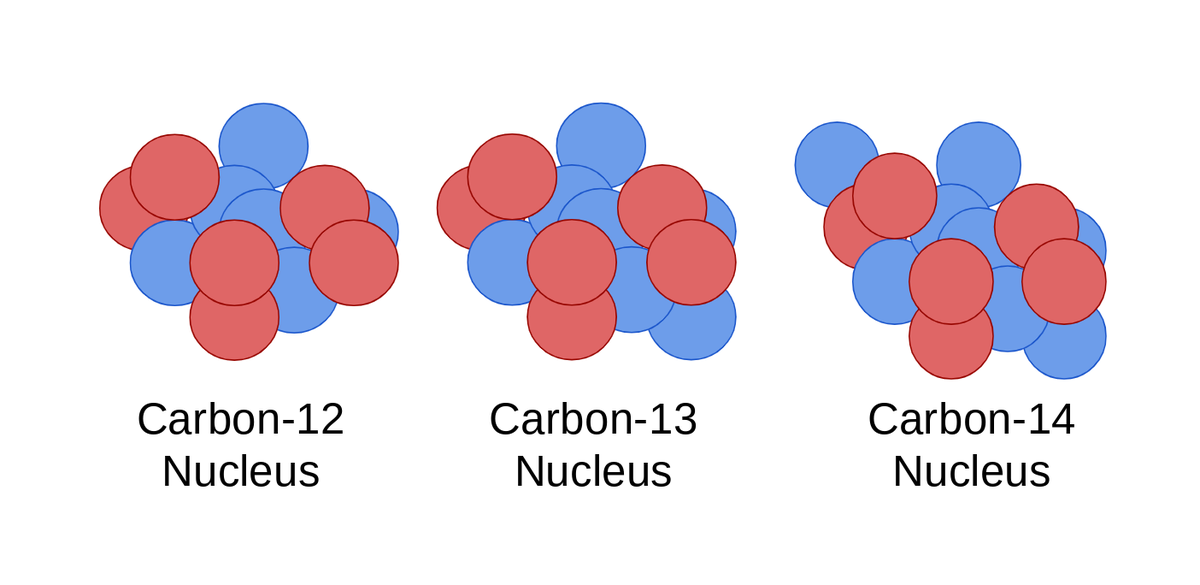
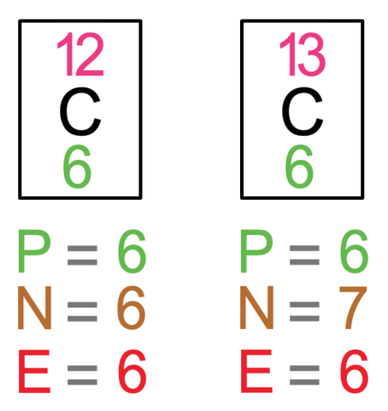
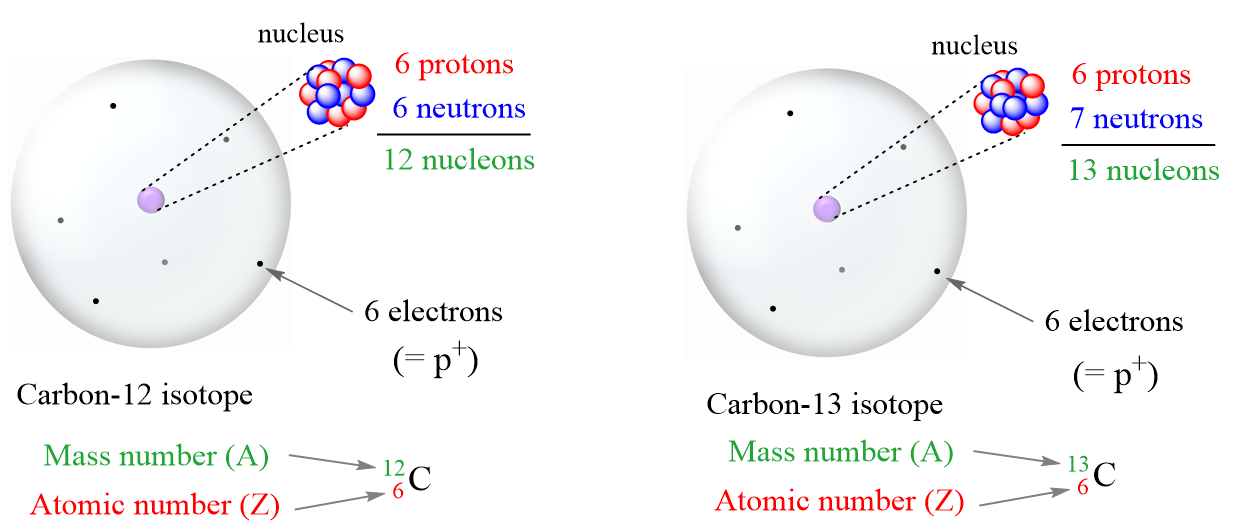
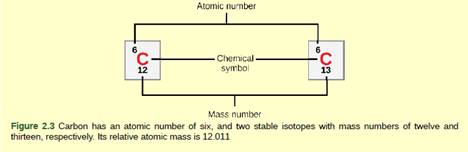
Kieran - Knowledge - The above image highlights the three naturally occurring isotopes of carbon: C-12, C-13, and C-14. So what is an isotope? An isotope is a form of an element

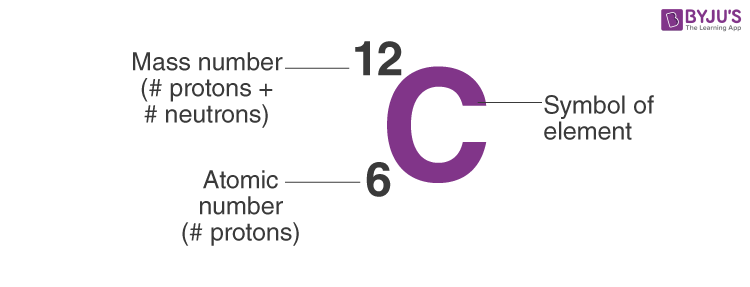
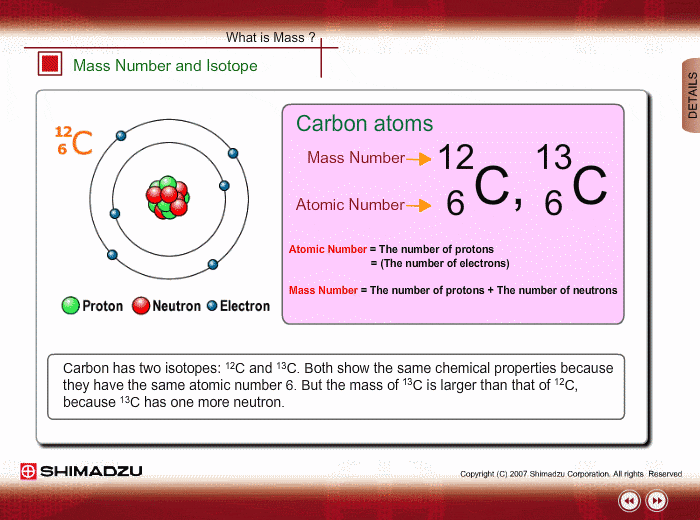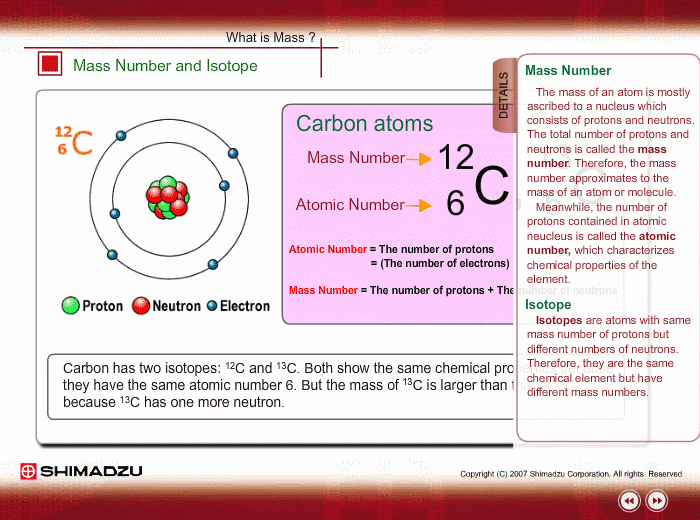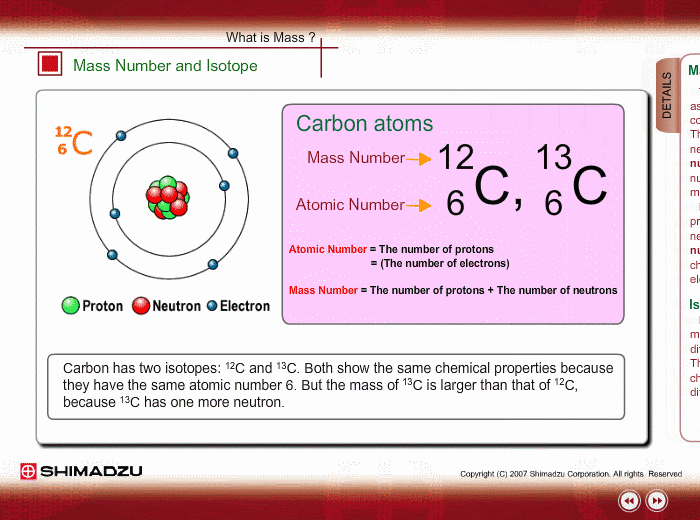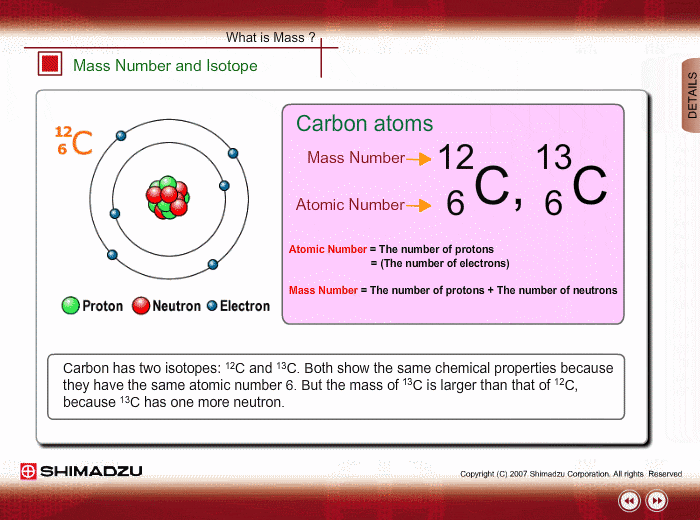
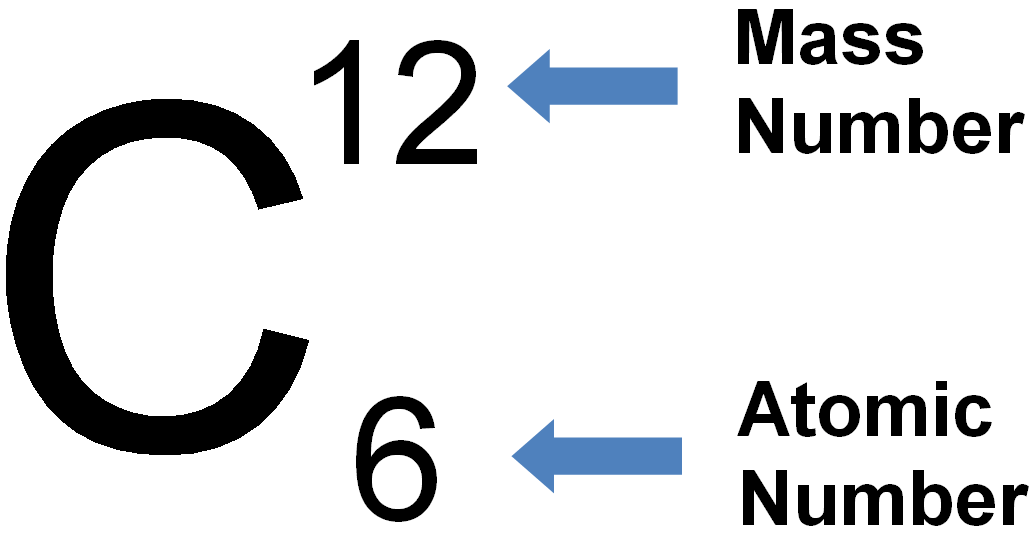
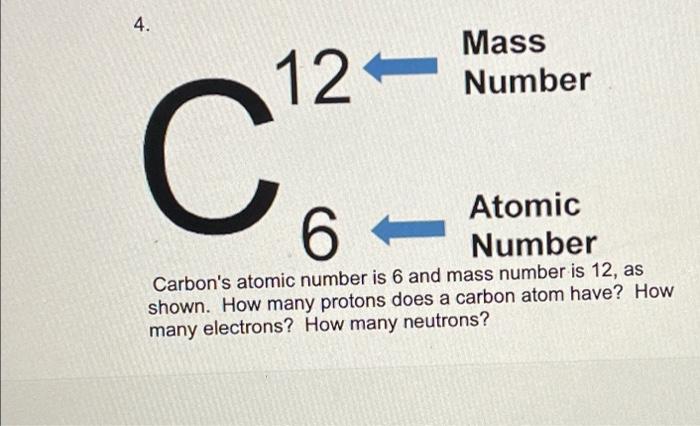
Atomic notation X A Z X = Symbol (C, Au) A = Atomic Mass Number = #nucleons (Protons + Neutrons) Z = Atomic Number = #protons C 12 6 Carbon A = 12, Z = - ppt download

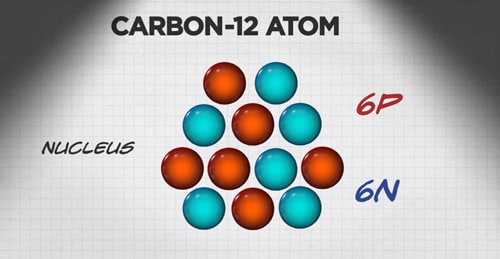
An atom of carbon has 6 protons. Its mass number is 12. How many neutrons are present in an atom of carbon ?