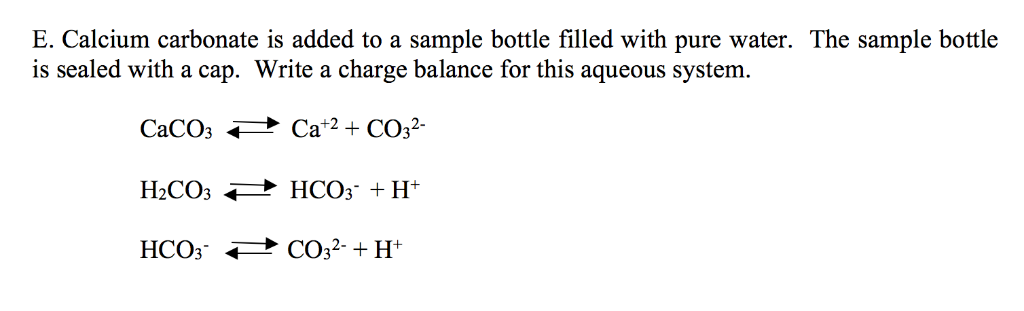Effect of pH on the surface charge of kaolinite and calcium carbonate... | Download Scientific Diagram

Calcium Carbonate-Modified Surfaces by Electrocrystallization To Study Anionic Surfactant Adsorption | Energy & Fuels

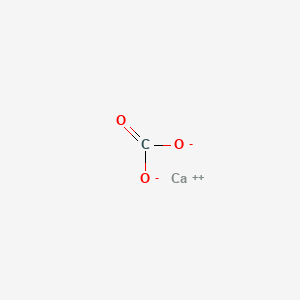
SOLVED: What is the charge on the cation in calcium carbonate? What is the charge on the anion in calcium carbonate?

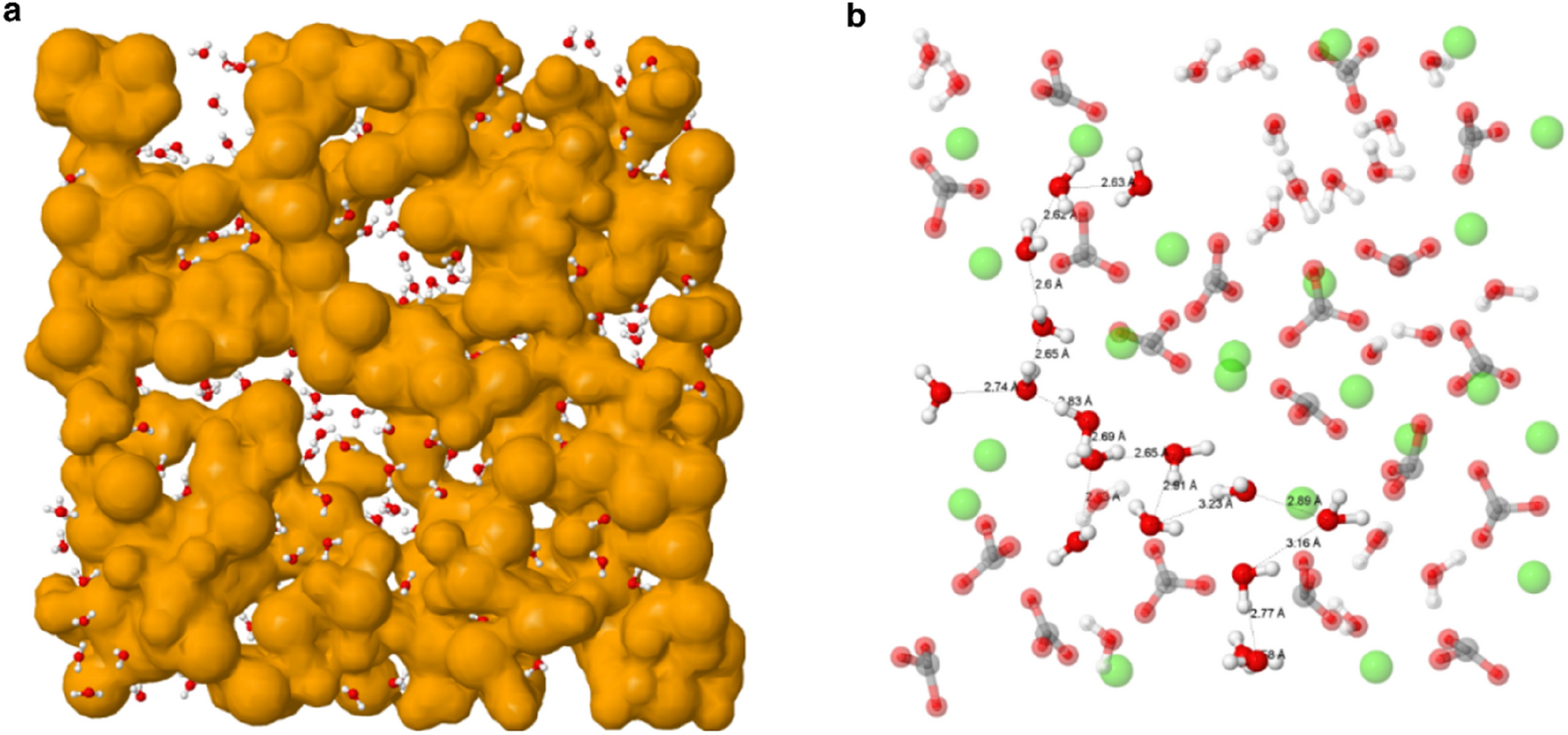
Oil-detachment from the calcium carbonate surfaces via the actions of surfactant, nanoparticle and low salinity brine: An insight from molecular dynamic simulation - ScienceDirect

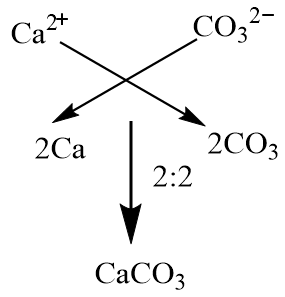
Here is the example in video form.. We're asked to write the formula for calcium carbonate Write the formula for calcium carbonate Ca 2+ CO 3 2– +2 –2. - ppt download
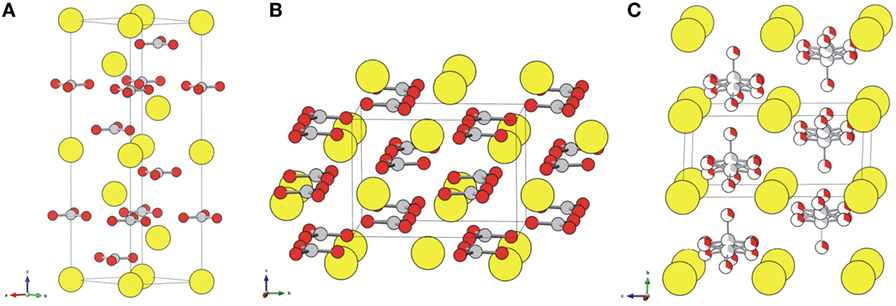
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism